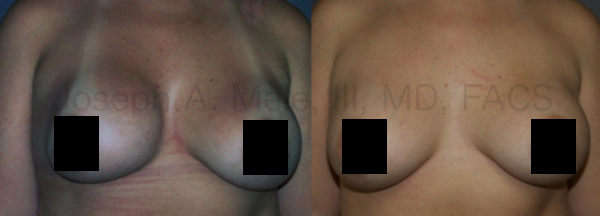
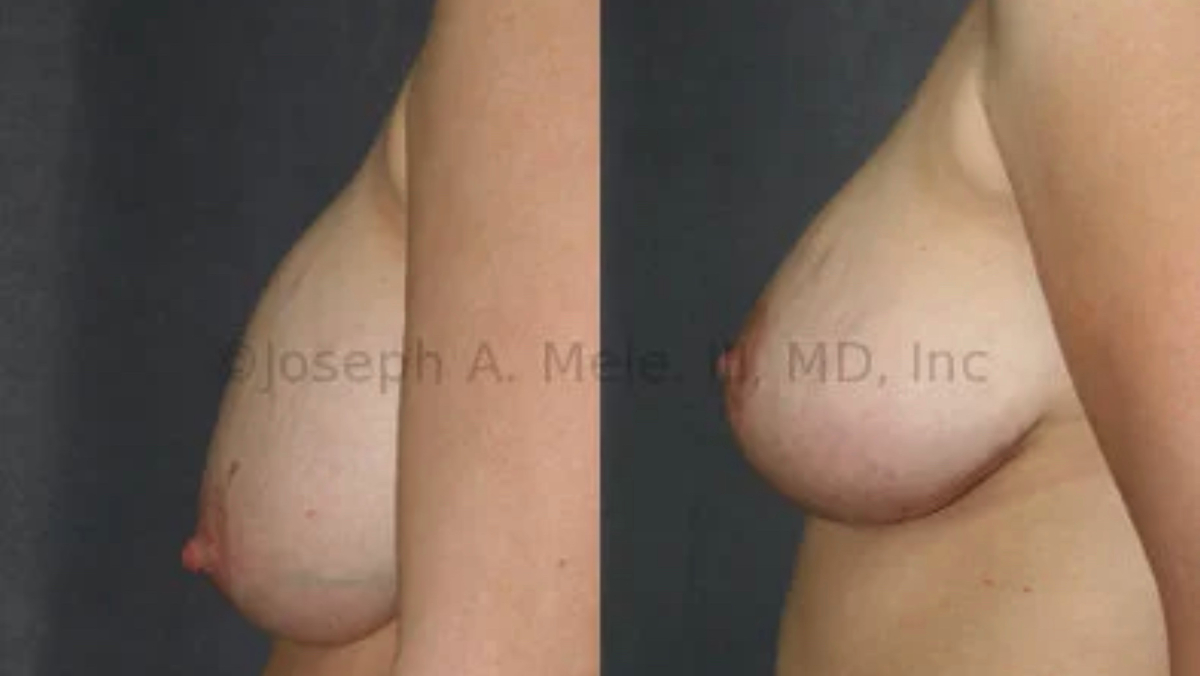
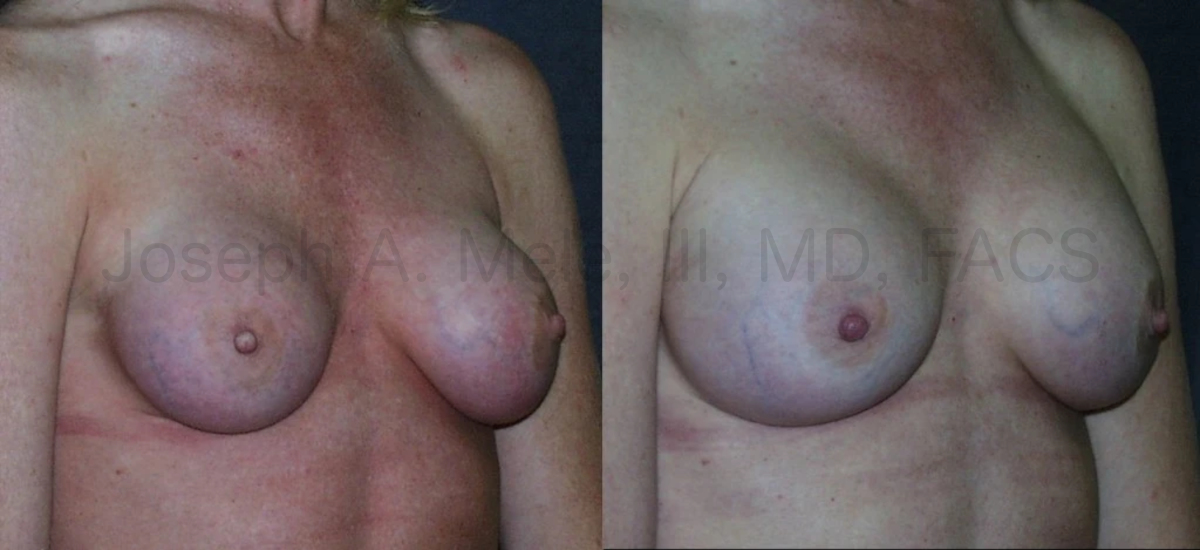
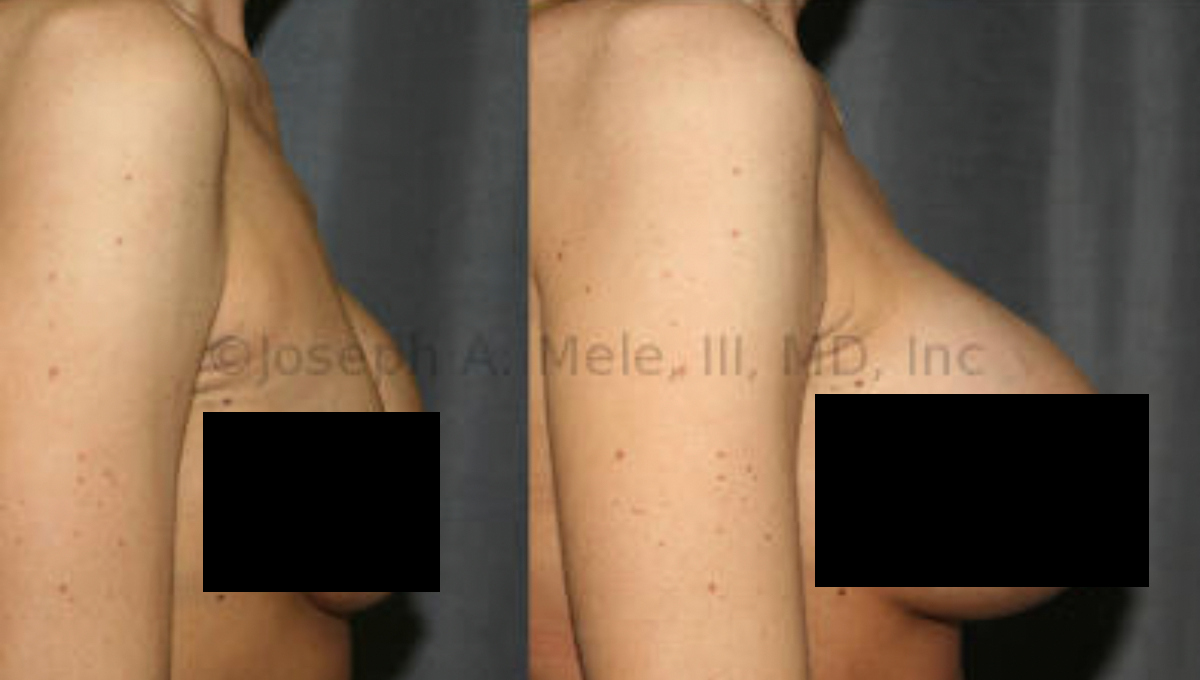
Downsizing Breast Implants – What You Need to Know
Breast Augmentation remains the most popular elective cosmetic plastic surgery procedure and has one of the highest patient satisfaction ratings. The ability to provide […]
Breast Implant Maintenance and Resizing
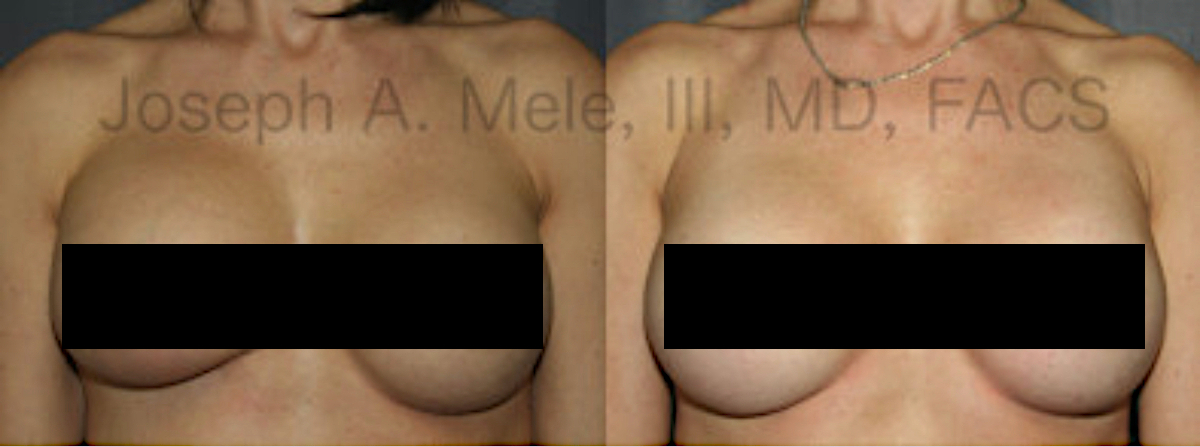
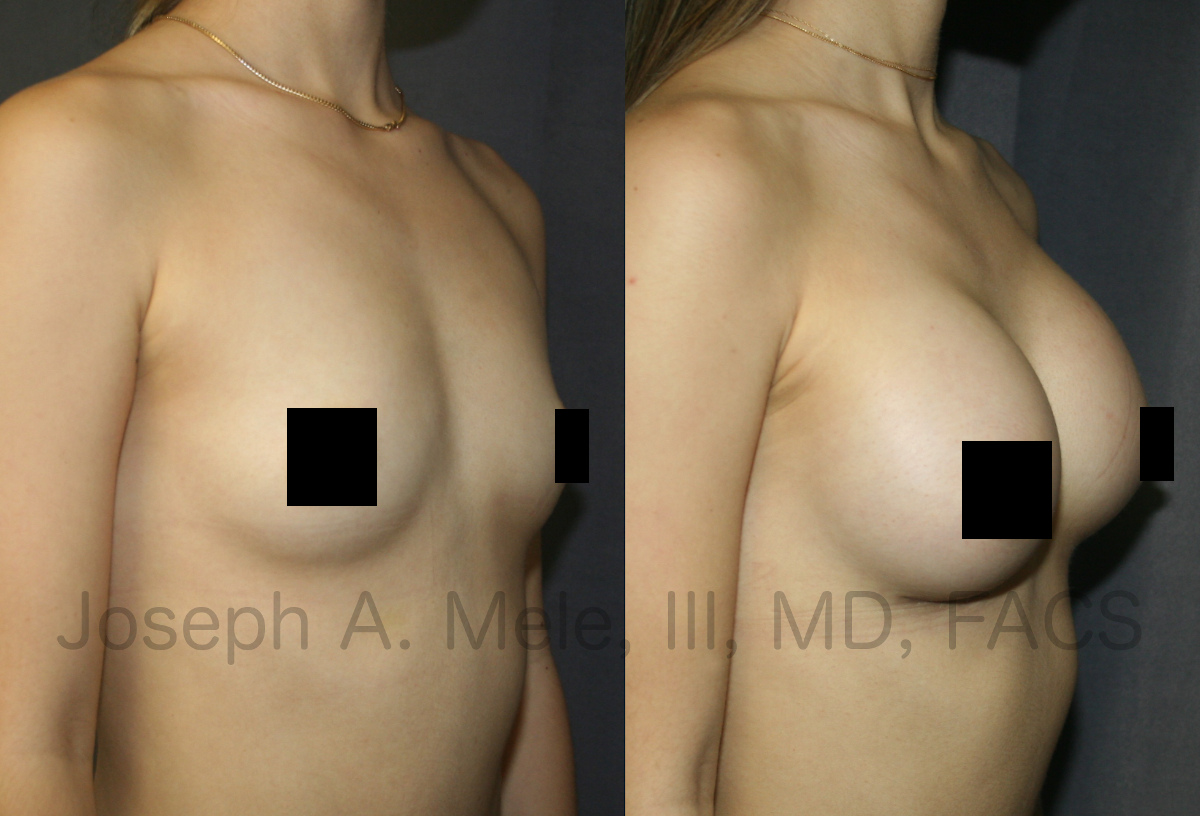
Breast Augmentation remains the most popular cosmetic plastic surgery. Whether placed for reconstructive or cosmetic reasons, Breast Implant Revision is needed from time to […]
Breast Cancer and Breast Implant Associated Cancer Update
In the United States there are about 270,000 cases of Breast Cancer each year, and 99% of them are in women. If you are […]
Breast Implant Maintenance and Breast Revision Surgery
Breast Augmentation Revision is used to maintain the aesthetic results of breast enhancing surgery. As we discussed last week, Breast Implants are not lifetime […]
Breast Implant Maintenance and Breast Revision Surgery
Breast Implants have come a long way over their 60 years of existence. They are available in a wider range of shapes, volumes and […]
Breast Implants and Mammograms
I subscribe to many feeds that feature the greatest and latest plastic surgery news. Much of it is smoke and mirrors, but every now […]
Breast Implants and Cancer of the Breast
The most common cancer found in the breast by far is primary breast cancer. This is cancer that originates in the ducts or glands […]
Why Choose Breast Augmentation Near Me?
Breast Augmentation continues to be one of the most frequently requested cosmetic plastic surgery procedures. While Breast Implants have changed, the goals of Breast […]
Breast Implant Replacement Surgery
A week doesn’t go by without someone asking, “When do I need to replace my breast implants?” The simplest answer is, “When there’s a […]
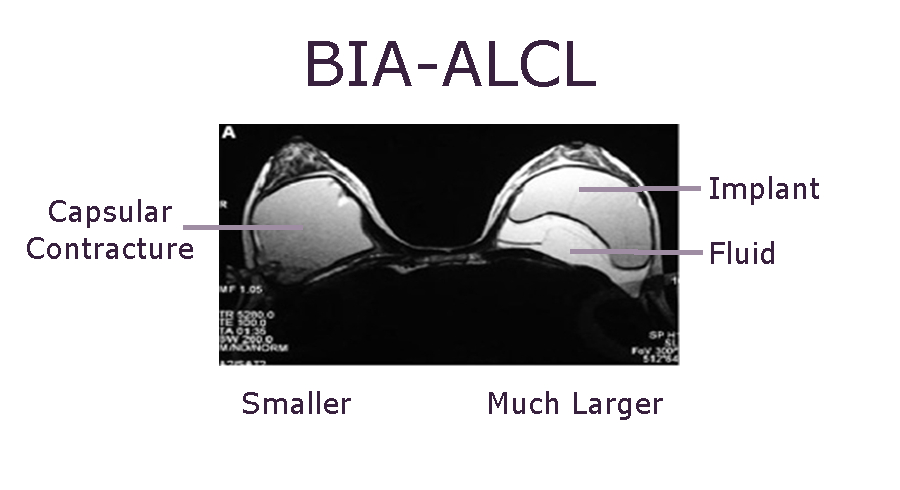
BIA-ALCL Update 2022
It has been 30 months since Allergan recalled its BioCell textured breast implants from the market. So what is the latest news regarding BIA-ALCL […]