It has been 30 months since Allergan recalled its BioCell textured breast implants from the market. So what is the latest news regarding BIA-ALCL (Breast Implant Associated Anaplastic Large Cell Lymphoma)?
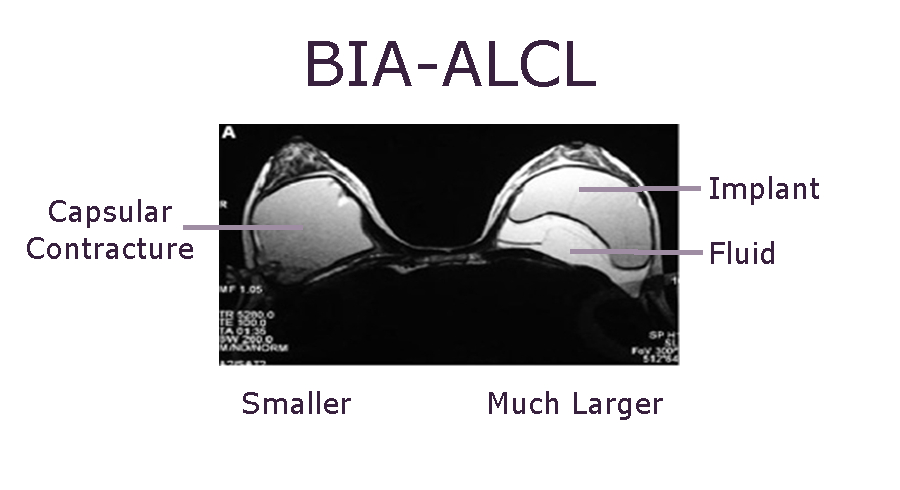
BIA-ALCL is often diagnosed by the disproportionate enlargement of one breast. The above MRI is an example of fluid around a breast implant which is suspicious for BIA-ALCL. The next step is sampling the fluid for analysis and definitive diagnosis.
Brief BIA-ALCL Background
On September 12, 2019, the FDA issued a Class I recall of all Allergan BioCell Textured Breast Implants, the most serious type of recall, due to an association with BIA-ALCL. While the overall risk of BIA-ALCL remains low, these specific implants were six times more likely to be associated with BIA-ALCL when compared to the other textured breast implants available at the time.
What Does the Recall Mean?
The most significant outcome of the FDA recall was the removal of all Allergan BioCell textured breast implants from the market. These breast implants are no longer available, and should not be used. If you have Allergan BioCell textured breast implants, the current recommendation from the FDA is: If you do not have symptoms, they do not require removal.
On the other hand, you should know the signs and symptoms of the disease. As long as you have your breast implant(s), you should monitor your breast area for any changes such as unilateral breast swelling (#1), a lump in your breast or armpit (less common), persistent breast pain (less specific) or a rash (rare).
Should I Have My Breast Implants Removed?
While BIA-ALCL is rarely the cause, any change in your breast implants should be investigated by your physician. You should discuss all benefits and risks with your board certified plastic surgeon. It is important to know the current risks associated with any surgery are higher than the risk of developing BIA-ALCL. So patients without any symptoms are not likely to benefit from Breast Implant Removal and it is still not currently recommended.
What Are The Symptoms of BIA-ALCL?
The most common sign of BIA-ALCL is fluid or swelling around a breast implant. This usually happens many years after the implant was originally placed. BIA-ALCL can also cause tumors that arise from the scar capsule around the implant. Less commonly, BIA-ALCL can cause a breast to become lumpy or misshapen with the development of a thick scar capsule around an implant.
How Is BIA-ALCL Treated?
If you have been diagnosed with BIA-ALCL, En-Bloc Resection is currently recommended. En-Bloc Resection is often a misused term. It is used only for cancer removal. In a BIA-ALCL diagnosed patient this includes removing the breast implant with a complete capsulectomy in conjunction with any associated mass and a rim or margin of surrounding healthy tissue.
There is no benefit to removing normal healthy tissue unless you have cancer. En-Bloc Resection is used in cancer treatment because tumor cells can invade the surrounding normal tissue, and removing a rind of normal cells increases the chances that all the abnormal cells are removed.
Is Prophylactic BIA-ALCL Treatment Available?
If you do not have BIA-ALCL, but you choose to have your breast implant(s) removed out of concern for BIA-ALCL, you should have a discussion with your surgeon about Breast Implant Removal, implant exchange, and partial or total scar capsule removal. The surgical removal of the scar capsule around your implant is called a “capsulectomy.” In an otherwise healthy patient, having a total capsulectomy at the time of implant removal is not known to change the risk of developing BIA-ALCL. This is a back-handed way of saying it provides no known benefit. The procedure, however, does have its risks. The risk of performing a capsulectomy includes, but is not limited to, bleeding, infection, loss of breast skin, wound complications, pneumothorax and the risks associated with anesthesia. For reconstruction patients, a capsulectomy could result in undesirable changes to the shape to your breast or loss of the reconstruction. While the risks of surgery are small, without any physical benefit, they may be higher than any psychological benefit provided.
How Do I Know If I Have BioCell Textured Implants?
If you have Sientra Breast implants or Mentor Breast implants, you do not have BioCell textured implants. If you have Allergan Breast Implants most of them are not textured. In my office, I rarely use textured breast implants, especially for round implants.
If you have the device information card provided to you after your breast implant surgery, the size, serial numbers, type of implants and date of your procedure is on the card. If you do not have the card, check with your surgeon or Allergan directly to see if your implants are textured. The operative report from the hospital or surgery center where your surgery was performed should also contain the information. You should know which breast implants you have. If you don’t know, get the information now; don’t wait.
How Do We Get BIA-ALCL Information?
The credit for the early identification, treatment and statistics on BIA-ALCL goes to your local Board Certified Plastic Surgeons and our two major national societies (ASPS and ASAPS). More information is available on the ASPS’s BIA-ALCL Resources Page.
Previous Post Next Post